
Photomicrography and Video of Protozoa, Volvox and Rotifers
by Dr. Robert Berdan
April 1, 2018
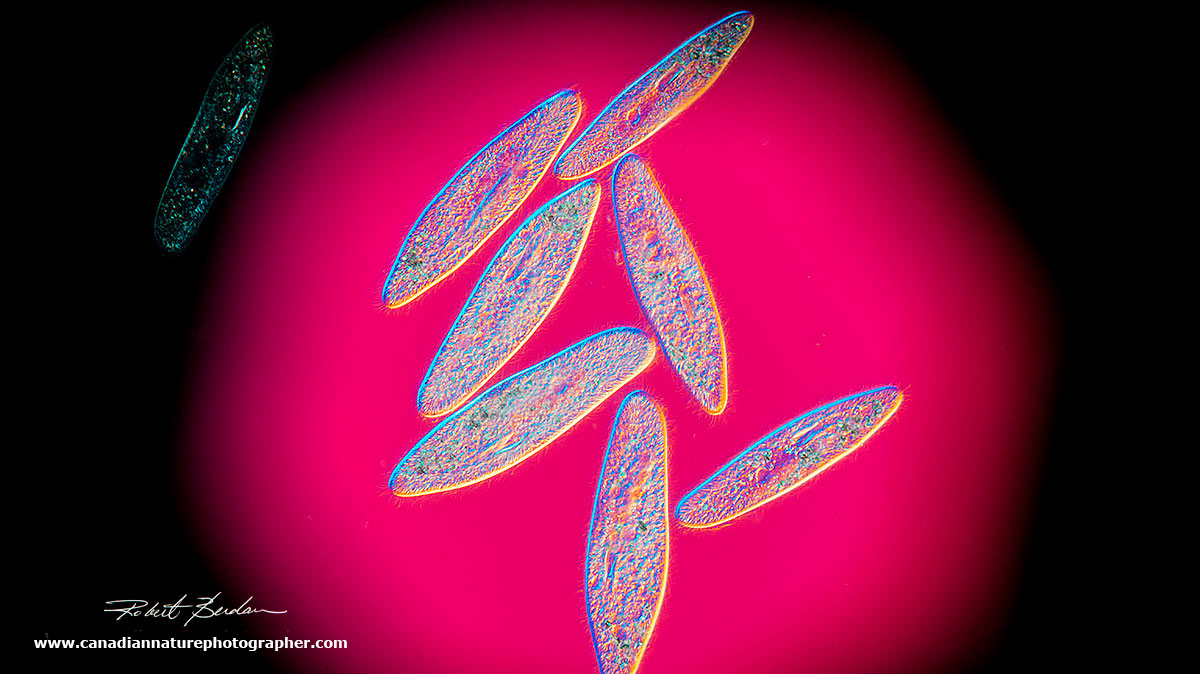
Paramecium gather in the circle of light which I produced by closing the iris diaphragm on the base of the microscope lamp. Darkfield microscopy 100X.
In the past year I have been setting up my home laboratory and getting my microscopes and macro-photography equipment set up to photograph specimens magnified from 1-1000X . I have owned an Olympus microscope for many years but I always wanted to own a microscope with Differential interference contrast (DIC) also called Nomarski Interference. A few months ago I purchased a Zeiss Axioscope microscope with DIC optics and I am thrilled. This type of optics provides microorganisms with a 3D-like appearance and it can colourize the specimens and background when used with a full wave plate. DIC microscopes are expensive, but provide some of best views of live cells and microscopic pond organisms.
In this article I show some of my recent photos of protozoa and rotifers with DIC microscopy. I couldn't wait for the ice to melt from the ponds in Calgary so I ordered some of these organisms from Boreal science. Boreal is a science supply company that sells invertebrates for teaching purposes. A test tube of single cell animals and plants costs $12.00 and is suitable for a classroom of about 30 students. Of course all these animals are available for free in ponds, ditches, roof gutters, bird baths and slow moving streams during warmer weather.

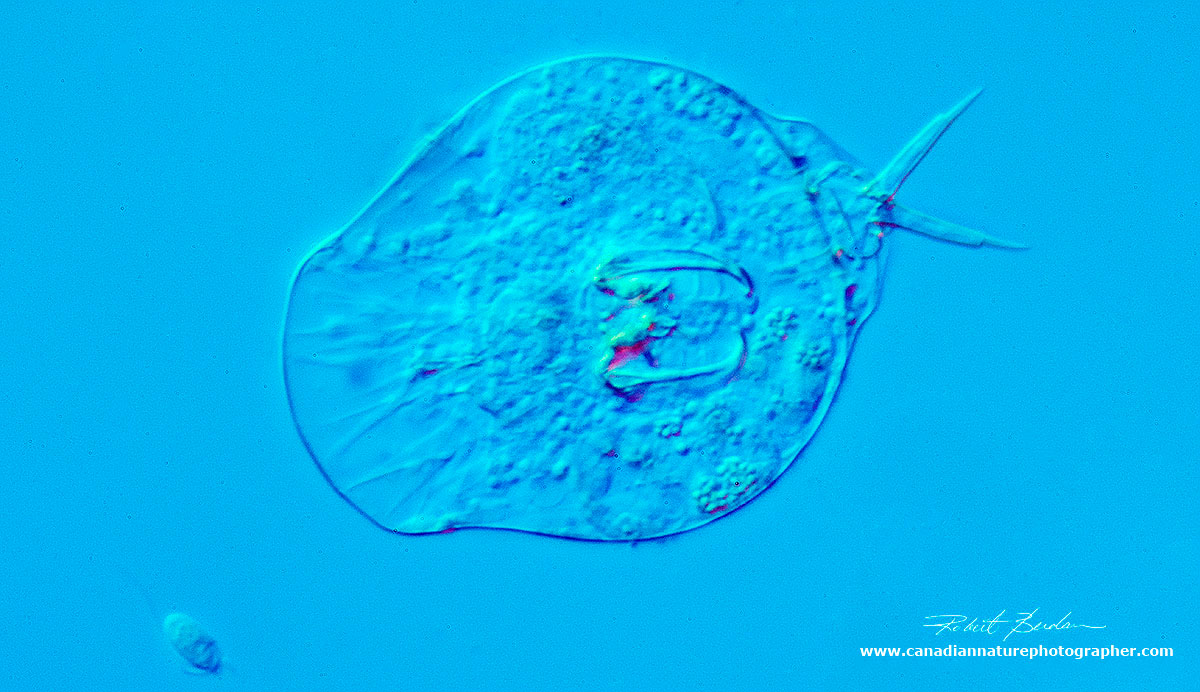
Rotifers are small multicellular organisms living in pond water that feed on other protozoa, bacteria and algae. This one is shown at about 400X using DIC microscopy and surrounded by 3 smaller euglena - flagellated protozoa. In the middle of the rotifer is a pair of jaws called trophi that move continuously to crush injested food. The trophi are important taxonimically. Rotifers are one of the most common animals found in pond water.
My goal is to show some of the complexity and beauty of a few microorganisms and hopefully bring them to the attention of a wider audience. When I was young I watched these organisms using a $30 Sears microscope and it inspired me to pursue a career in cell biology and later to become a professional nature photographer. A single cell protozoan can move, hunt for food, reproduce asexually and sometimes find a sexual partner. Many of them can form cysts and go into suspended animation (anhydrobiosis) for months or years when their pond dries up. In short, they are some of the most perfectly adapted animals on the planet.
We have much to learn from Protozoa and Rotifers. They are all around us and a visit to any outdoor source of water even rain water from roof gutters will have hundreds if not thousands of different species. Along with bacteria, they form the base of the food chain and break down animal and plant matter. We could not live without them. They are also some of the most fascinating and unusual animals I have ever encountered, some do not age (Hydra), others can exhibit incredible regenerative abilities and even exhibit simple forms of learning and yet protozoa are just single cells without a brain. They fascinate me and I hope you might want to see them for yourself - of course you will need a microscope but they are all around us. Used microscopes or even new microscopes can be purchased for a few hundred dollars on Kijjii, ebay, government surplus and various online microscope stores.
Paramecium caudatum
There are at least 10 species of paramecium. These one celled ciliates live in fresh water and are common. They can be cultivated and induced to conjugate (exchange genetic material). Research studies show that paramecium can divide about 200 times before showing signs of aging, however after conjugation they are renewed and young again. Research on paramecium suggests that aging is due to DNA damage over time and not due to changes occurring in the cytoplasm.

Paramecium caudatum viewed by Differential Interference microscopy (DIC) with a waveplate at about 600X
Paramecium vary in size from 50-300 microns (one micron = 1\1000 of a millimetre) and there are many similar ciliates you will encounter when collecting samples from pond water. The name paramecium means "oblong" shaped. They swim using cilia (fine hairs) which covers almost their entire body. When they hit something they back up, turn and then move forward again, the so called "avoidance reaction". To get sharp pictures I had to wait until the water under the coverslip of my microscope slide evaporated and pressed down on the animals holding them still. Paramecium feed on bacteria, algae, and yeast in the water.
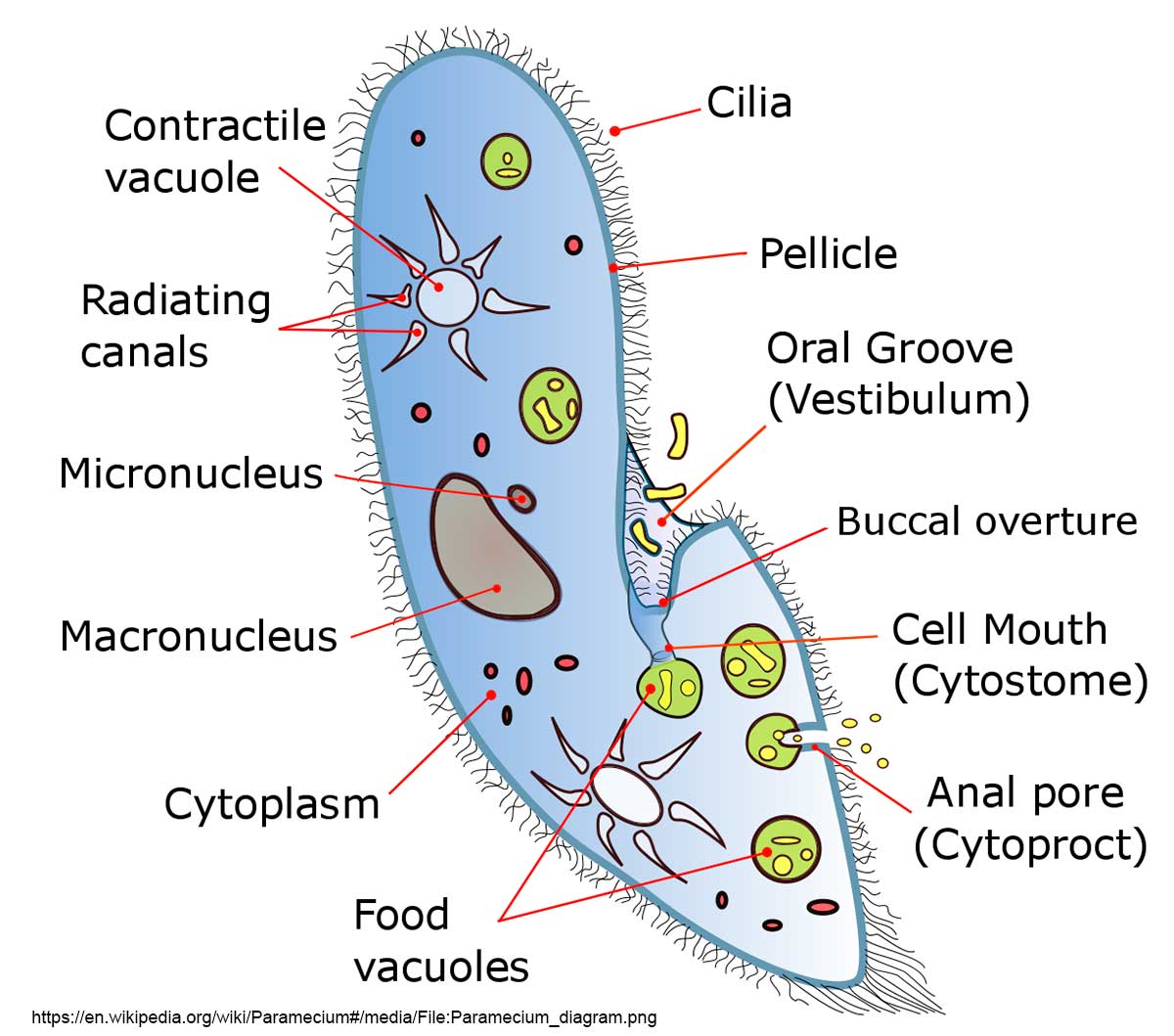
Diagram of Paramecium courtesy of Wikipedia. Try to identify some of these components in the pictures below.
When I was second year biology student at the University of Western Ontario, I was fortunate to meet Dr. Tracy Sonneborn, one of the leading researchers in Paramecium biology. I asked him for permission to attend one of his laboratory sessions reserved for graduate students and he agreed. In the laboratory he showed us various mutants he had produced and his enthusiasm was infectious. These are my first photographs of paramecium using a DIC microscope. I also have a photograph below with Phase contrast which shows quite a bit, but not as much as DIC.
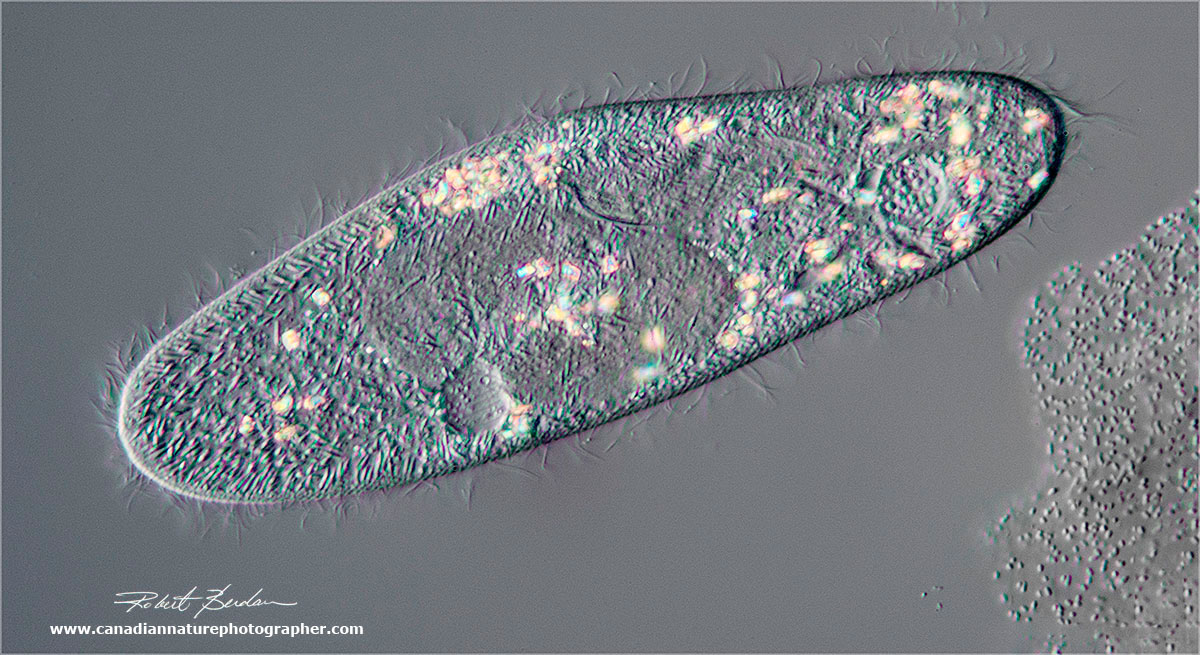
DIC microscopy of a single paramecium, showing some birefringent material inside the cell. On the lower right of the photograph are some bacteria. 400X.
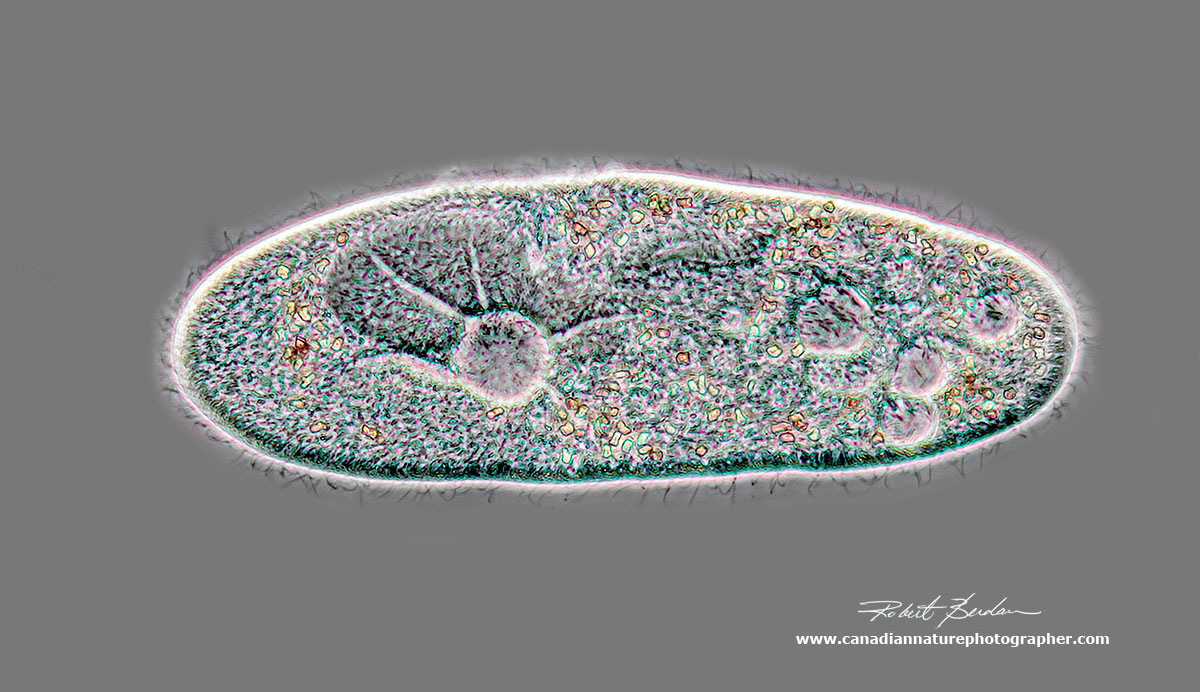
Paramecium caudatum viewed by Phase contrast microscopy - about 400X. The contractile vacuole is easy to spot on the left and functions to expel water and maintain osmotic pressure in the animal. Each animal has 5.000 to 6,000 cilia, about 0.25 microns in width and about 5-6 microns in length. Paramecium can travel up to 1000 microns per second (1 mm/sec).

In DIC microscopy I can vary the colour of the background and change the colours of the specimen to view the internal components of the Paramecium and it possible to clearly see the cilia which propel paramecium. 400X.

Using DIC and a full-wave plate I photographed this paramecium using an orange coloured background. 400X

Paramecium gathering in the light region of the microscope slide as shown above. 200X
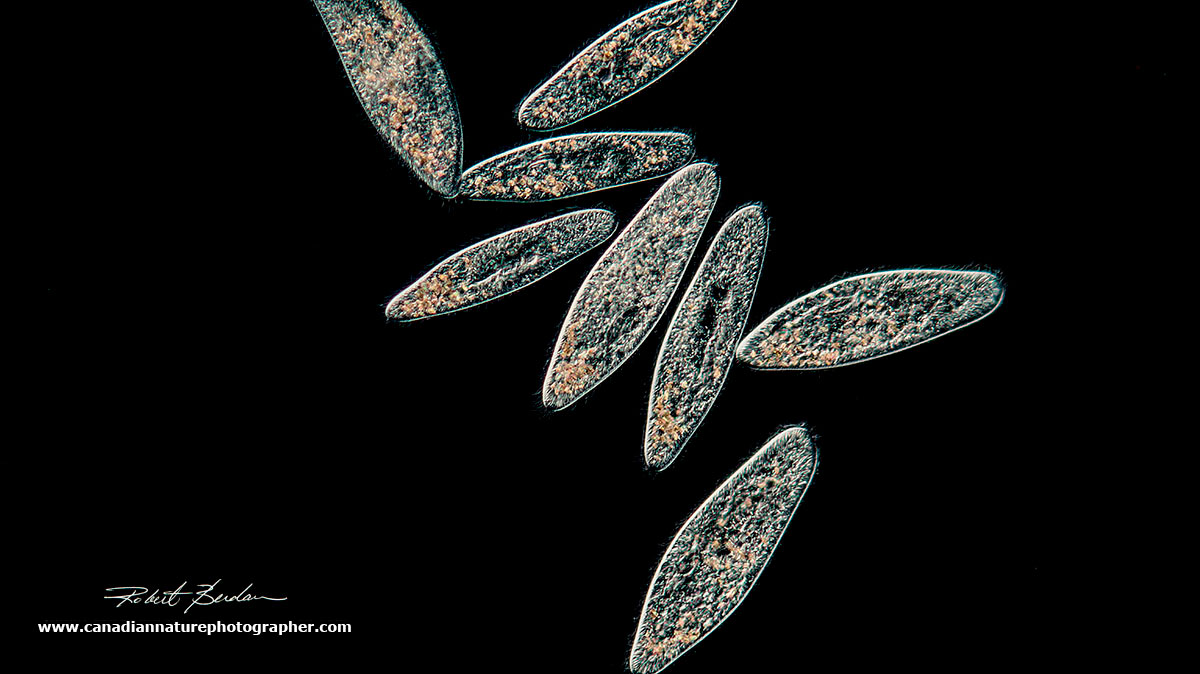
Paramecium via Darkfield microscopy. 200X
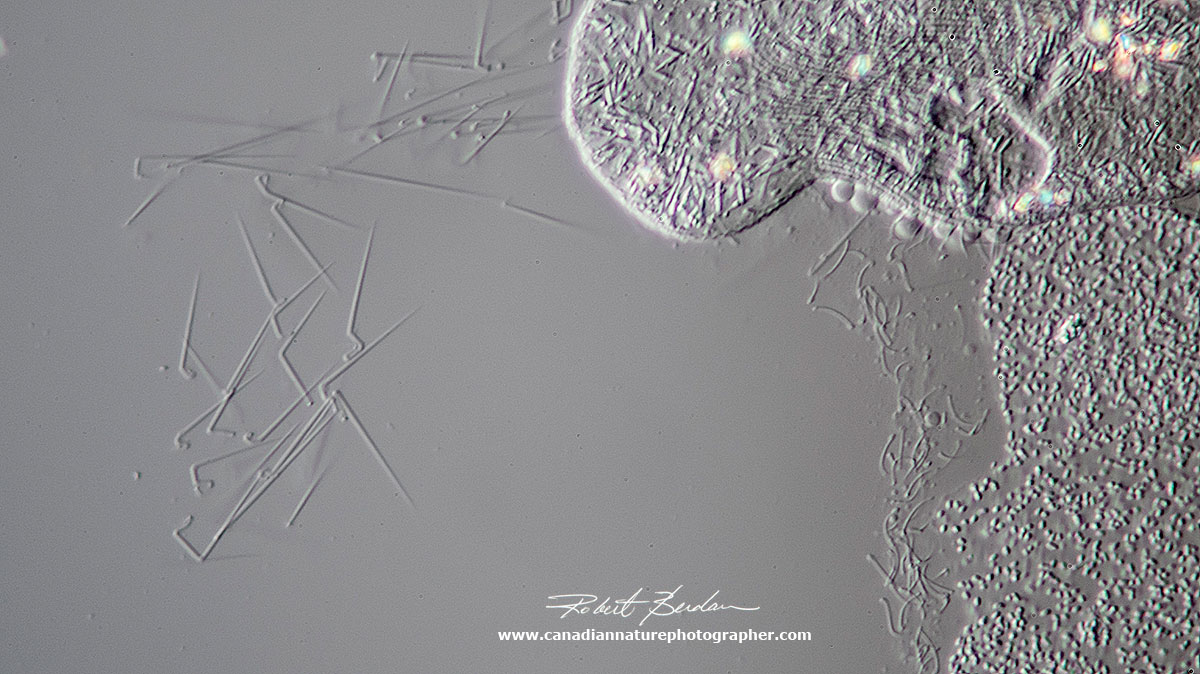
When paramecium are injured, come into contact with noxious chemicals, or are preyed upon they may release trichocysts - short needle like structures shown on the left side of the photograph. The function of trichocysts is uncertain but they may play a role in defence though they are not always effective.
My photographs show static images of the paramecium, but when viewed live or in a video below they can be seen to move quickly, forward, backward and often rotate and spiral as they swim.
Amoeba proteus
Amoebas are single celled protozoa that move by flowing protoplasm that form pseudopods (false feet). There are many kinds of amoeboid cells some belong to slime molds, algae and other organisms. Some of our own white blood cells e.g. macrophages resemble amoebas. The appearance and structure of the pseudopods are used to distinguish different groups. Some freshwater species also live inside hard shells (Arcella) and a few species can cause disease e.g. Naegleria flowleri found in warm bodies of water, soil, and unchlorinated swimming pools - this species can cause a brain disease which is often fatal, though infections are rare.
Freshwater amoebas have a contractile vacuole which expels water from the cell and regulates osmotic pressure. Amoeba move by extending their pseudopods and protoplasmic flow. They eat bacteria, diatoms, detritus, and other smaller protists. The ingest food by phagocytosis, extending pseudopods to surround and engulf their prey. They don't have a fixed mouth.
Some Amoeba have a single nucleus and others multiple nuclei. They are found at the bottom of ponds, they are also common in my gutters. I sometimes use a centrifuge to concentrate them from water I collect from my roof gutters. They also vary in size from about 20 microns to over 500 microns. When I place water samples on the microscope slides I usually wait a few minutes for the Amoeba to attach to the glass and start moving.
.jpg)
Diagram of a single celled Amoeba that lives in pond water. Diagram courtesy of Wikipedia.

Amoeba 400X as viewed by Differential Interference Microscopy (DIC) microsopy. Refer to the diagram to see what components of this cell you can identify. This was a smaller amoeba I found in the culture from Boreal science.
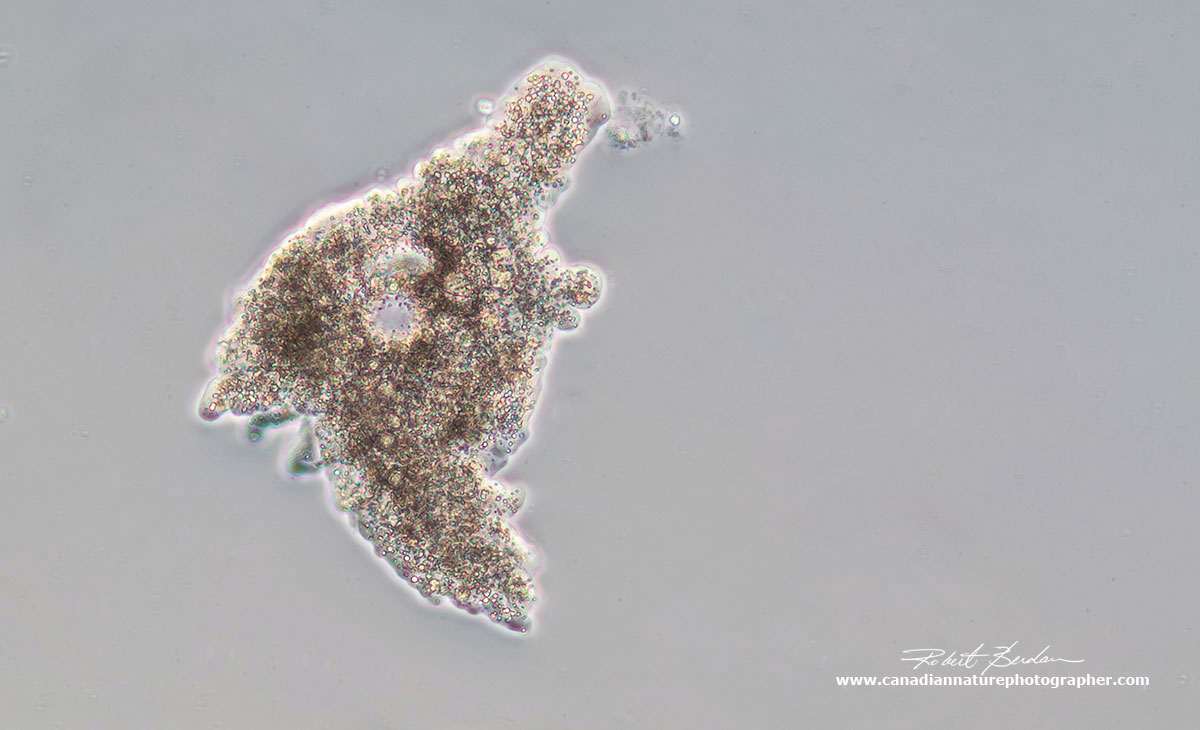
Amoeba proteus via DIC microscopy about 100X.

Wild Amoeba collected during the summer in a Calgary pond. Phase contrast microscopy about 150X Olympus microscope. The nucleus is easily seen as well as many small pseudopodia.
Stentor coeruleus
The name Stentor is in reference to its trumpet shape and the herald of Greek mythology known for having a loud voice. The species name "coeruleus" describes the blue-green pigmented stripes (see picture below). They grow up to 3 mm in size and are one of the largest single celled organisms. It has amazing regenerative capacity and this single cell has the ability to learn. Fragments of a single cell can regenerate into an entire new organism so long as the fragments contains part of the ribbon shaped macronucleus and portion of the membrane/cortex. Stentors are common in fresh water ponds and swim using cilia that cover its body surface and they also can change shape from a long trumpet into a sphere.

Diagram courtesy Research Gate. All of the features shown are on the cell surface, except for the macronuclear nodes, the micronuclei, and the contractile vacuole, all three of which are located just beneath the surface. The cortical fibrillar system, not shown in the diagram, is located within the clear stripes between the granular (pigmented) stripes.
The cilia in the oral region create a vortex sweeping in food, mostly bacteria and other smaller ciliates and algae. Some animals have algae living inside that form a symbiotic relationship. It's not understood why they algae "Chlorella" are not digested. Stentor is able to exhibit a simple form of learning called "habituation" where the animal learns to ignore a mechanical stimulus and retains this "shot term memory" for several hours.
The genome of Stentor has recently been sequenced and scientists hope to learn more about how this animal is able to regenerate (stentor.ciliate.org). The large macronucleus is polyploid, which means it has many duplicated copies of genes. Many different types of single cells that are very large and able to regenerate are polyploid. So far 12 species of Stentor have been described.
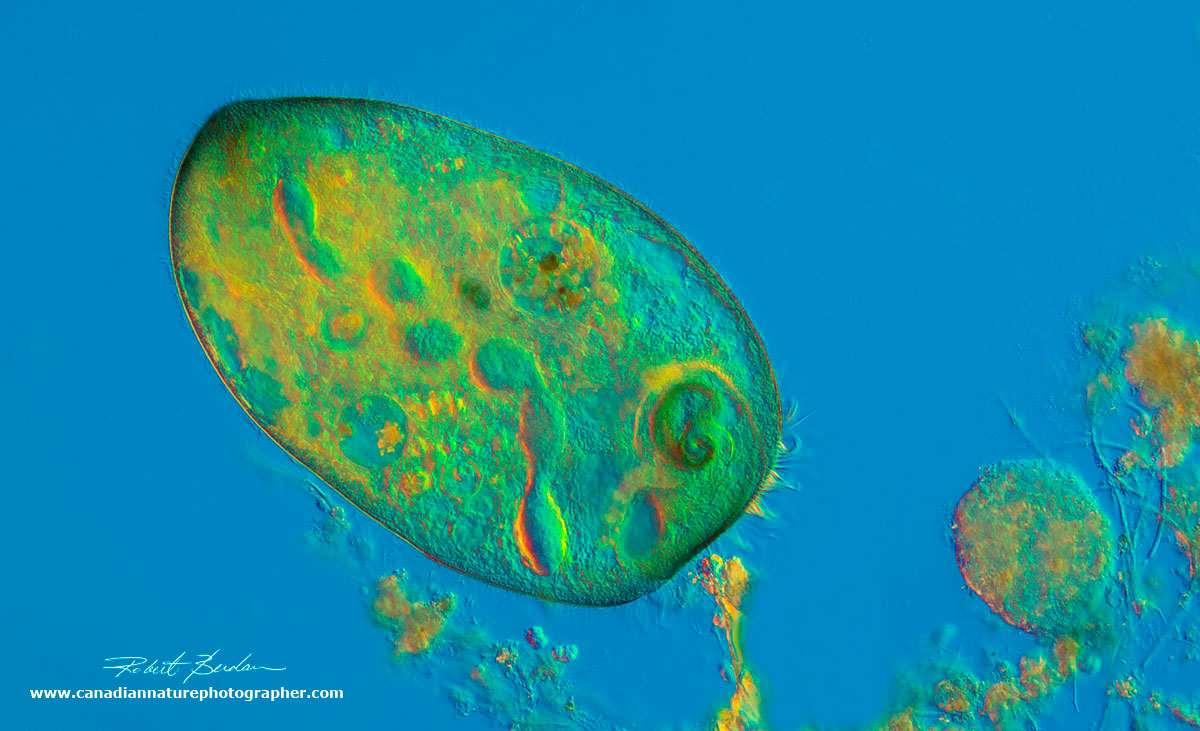
Stentor coeruleus searching for food. You can see part of the macronucleus, and some cilia around its anterior end. 100X.

Stentor coeruleus, the anterior end is at the top left and the foot is at the lower right. The organism can form a ball or stretch into a long trumpet shape. They feed on bacteria, algae and other smaller protozoans. Differential interference microscopy (DIC) about 100X.

DIC microscopy of Stentor about 50X DIC microscopy.

Rheinberg and DIC lighting of Stentor which has contracted into a spherical shape 100X

Surface of Stentor showing the pattern on the cortex. The clear stripes represent bundles of microtubules known as Km fibers, whereas the blue-green stripes contain dense fields of pigment granules.

Stentor from the wild I photographed using Phase contrast microscopy with my Olympus microscope in the summer of 2017. This Stentor has numerous intracellular symbiotic algae - Chlorella. 400X Phase Contrast Microscopy.
Euglena gracilis
Euglena have both animal and plant properties. They contain chlorophyll and can make their own food, but in the dark they can survive by feeding on bacteria. They swim about using a long flagellum and some have a red eye-spot that is photosensitive and they move toward the light (phototaxis). In fact all euglena have 2 flagella, but in some species the 2nd flagellum is very short and does not protrude from the cell. There are 54 genera and about 800 species. Sexuality seems to be absent in this animal as they reproduce asexually. The have an outer pellicle which has a helical screw symmetry.
Euglena can also take up food directly from the water by osmosis. They are widely used in research as they are easy to culture. In 2005 a Toyko based Euglena company started making food and beverage products from this organism and are also investigating its potential use in the formation of biofuels.
They can have 6-12 choloroplasts, a red eye spot which confers the ability to move toward light. The word Euglena means "eyeball organism" in Greek. They have 45 chromosomes and in 1994 DNA analysis indicates they have a number of unclassified genes that make new forms of carbohydrates. They are often found in ponds and ditches containing rotting leaves. Like many other protists they can form cysts in low moisture or when food is scarce.

Diagram of Euglena - a protozoan that can move yet it also has chloroplasts to produce its own food from light. It can feed on bacteria if cultured in the dark - it posses both animal and plant properties.

Two Euglena at the top showing a single flagellum it uses to move. The larger object is a part of a rotifer. 400X DIC
Euglena about 600X DIC microscopy.
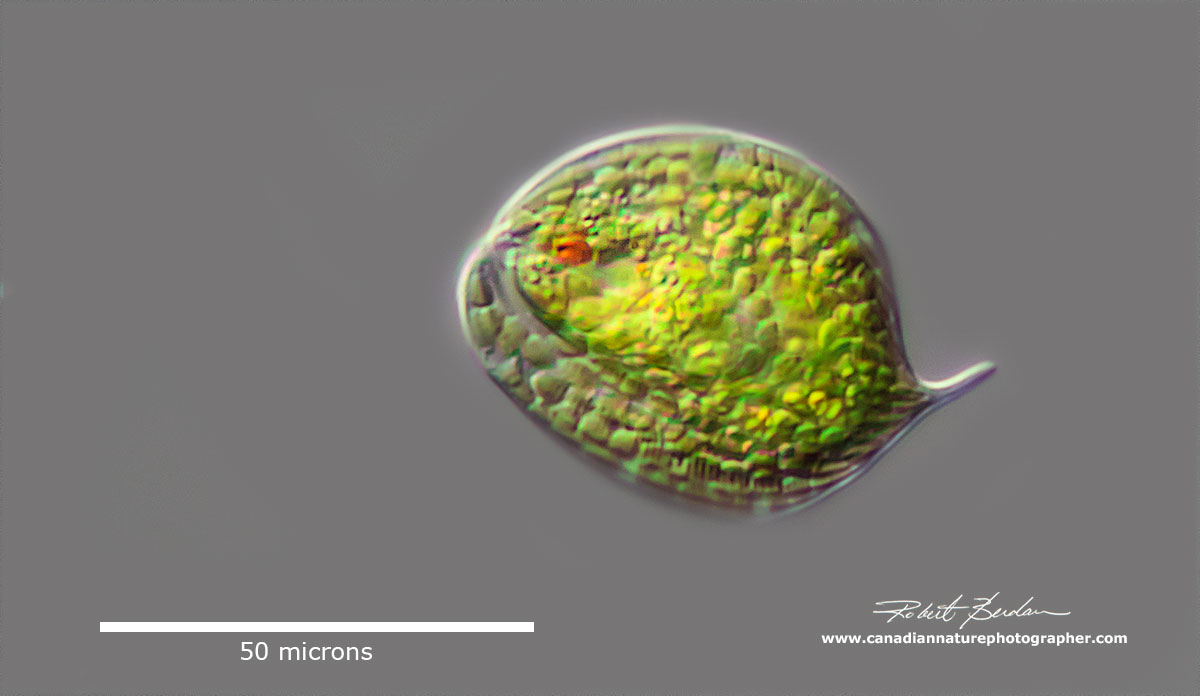
Phacus sp belongs to the phylum Euglenozoa, characterized by its flat, leaf-shaped structure, and rigid cytoskeleton known as a pellicle. There are currently 564 species of Phacus. DIC microscopy - photostack.
Vorticella
Voticella are bell-shaped ciliates that form stalks. The stalks have contractile myonemes that form after a free-swimming stage. The stalks can contract quickly into a coil to avoid predators. The name Vorticella is derived from beating cilia at the top of the bell that forms vortices that draws bacteria into their "mouth". They were first described by Anton van Leeuwenhoek in 1676. Most Vorticella are found in fresh water attached to plants, debris and even other animals e.g. copepods. Rotifers have been known to feed on Vorticella. They may also contain symbiotic algae "Chlorella". They have a large curved C-shaped macronucleus and smaller round micronucleus nearby. They also have contractile vacuoles which expel water that move into all freshwater protozoa. They divide by binary fission. There are about 200 species of Vorticella.
Vorticella have been discovered to inhibit the development and emergence of mosquito larvae and they are being explored as a method of biocontrol for mosquitoes which can spread disease.
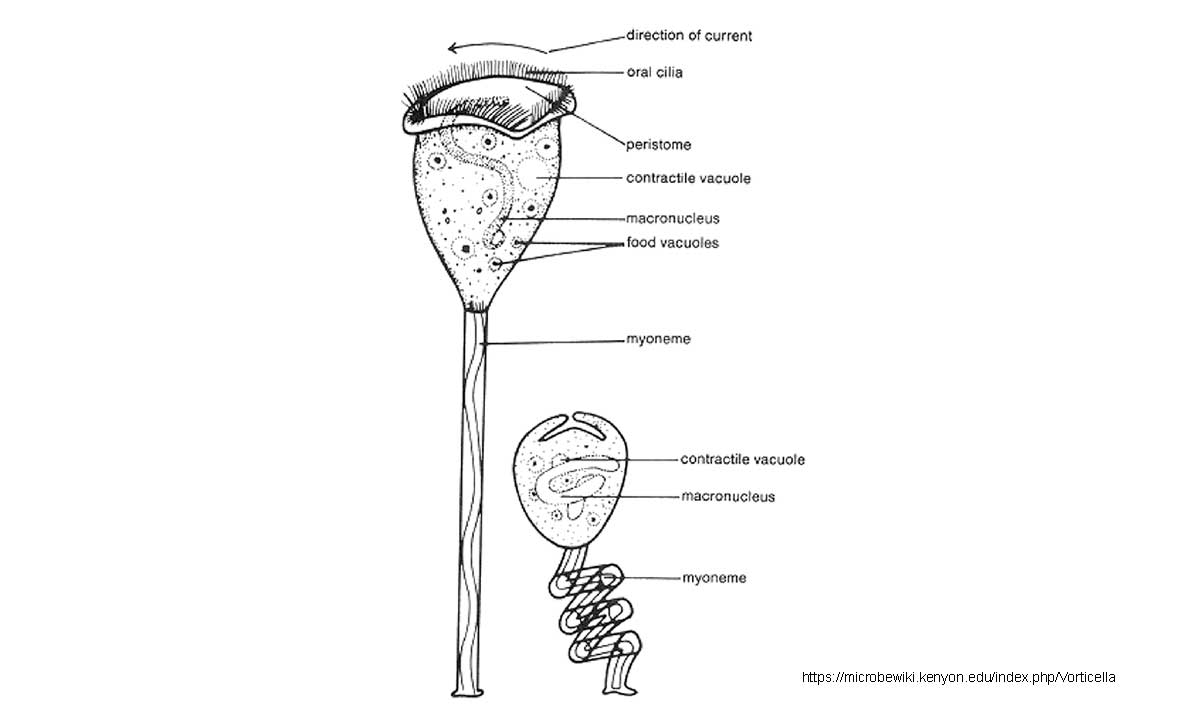
Diagram of Vorticella in the expanded and contracted state: Image source: https://microbewiki.kenyon.edu/index.php/Vorticella

Vorticella as viewed by Darkfield microscopy. The ciliary hairs at the top are moving so quickly they are blurred in this photograph. 250X.

Vorticella showing the long stalk. There is an outer tube and an inner filament (myoneme) which can contract quickly. 200X DIC microscopy.

Some Vorticella can lose their stalk and swim about. 400X DIC microscopy.

Vorticella at high magnification (600X) with DIC microscopy. The large backward C shape is the macronucleus, ciliary hairs can be seen at the top. The Vorticella is anchored by long stalk that can contract quickly.
Volvox aureus
Volvox forms spherical colonies containing up to 50,000 cells and form a hollow ball. It's made up of individual flagellated somatic cells and smaller number of germ cells. There are 23 different species. Because the cells contain chlorophyll it's classified as a form of algae. The cells can swim in a coordinated fashion, and they each have a red eye-spot that is sensitive to light and the colony will move towards light. Volvox can reproduce sexually and asexually. Heat stress can induce them to reproduce sexually. The cells are for some time, interconnected by cytoplasmic bridges that form a syncytium.
Volvox is found in freshwater ponds, ditches and shallow puddles that receive an abundance of rainwater. In some you can see smaller ball of cells inside which are the daughter colonies. These daughter colonies are inside out at first with the flagella facing inside, they need to invert before being released. The inversion process takes about 40 minutes and they eventually break through the adults outer shell.
It's believed the single cells transitioned to form colonies at least 200 million years ago.
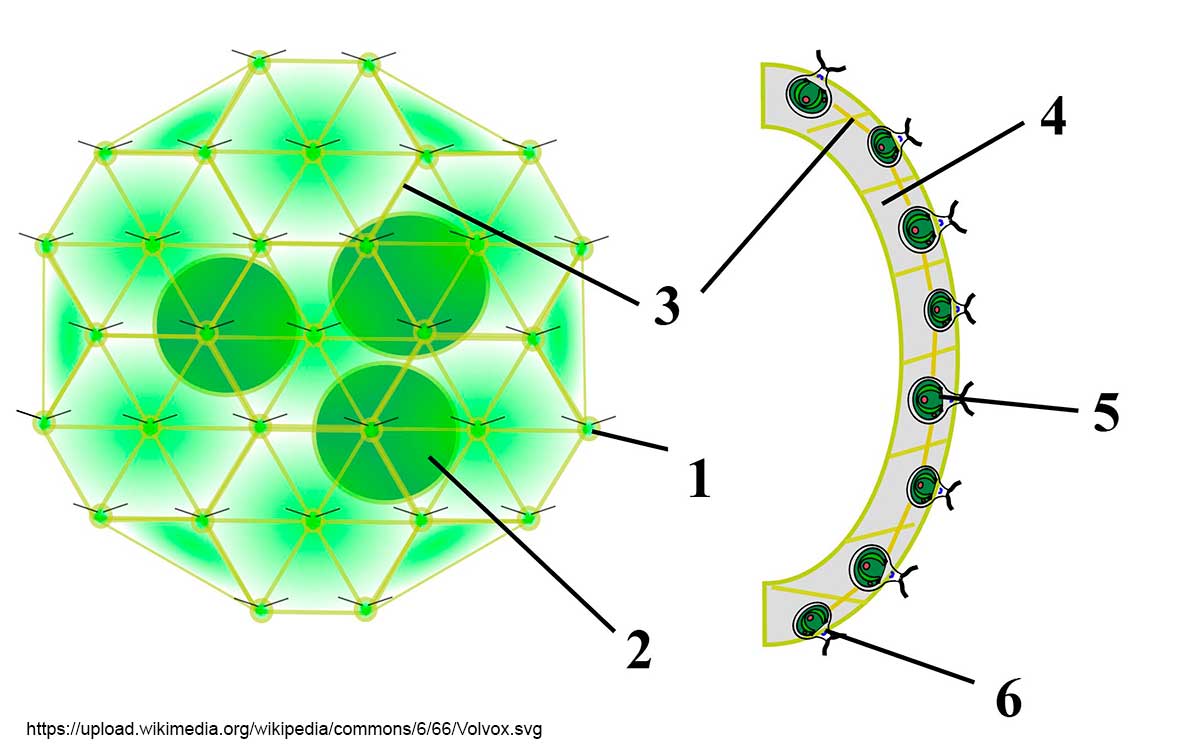
Diagram from Wikipedia by Sundance Raphael. Volvox colony: 1) Chlamydomonas-like cell, 2) Daughter colony, 3) Cytoplasmic bridges, 4) Intercellular gel, 5) Reproductive cell, 6) Somatic cell.

A Volvox colony, DIC microscopy about 300X. The individual cells can be clearly seen. Focus stack.
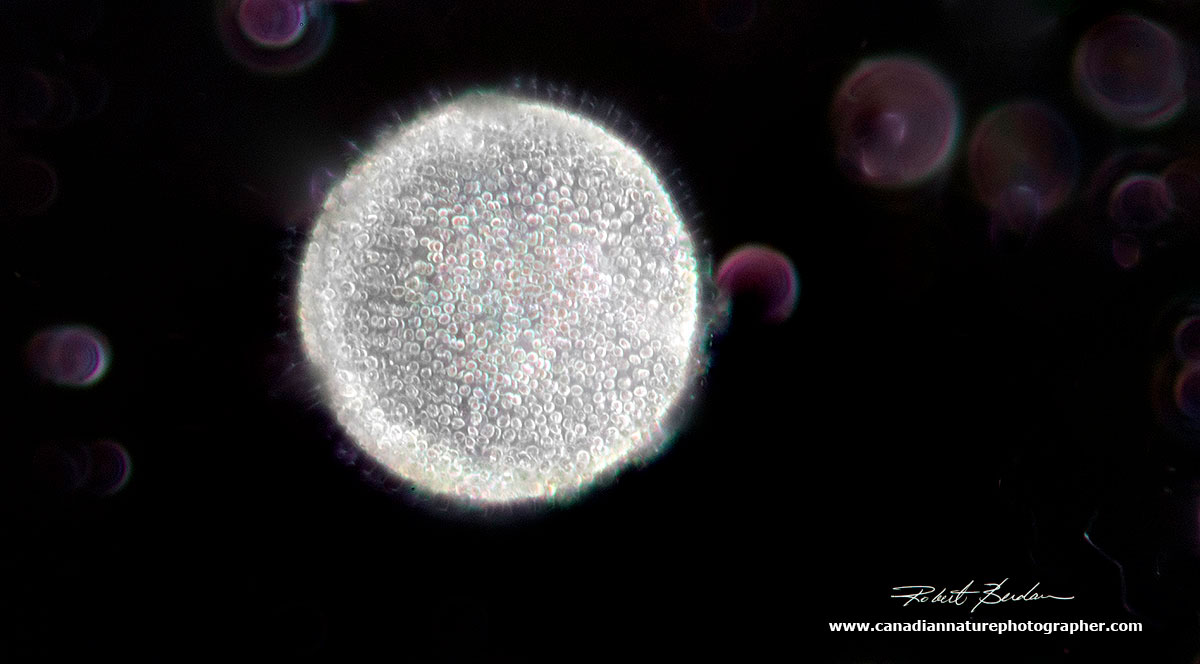
With darkfield microscopy this Volvox looks like the sun in outer space. The purple circles are out of focus ciliates. 200X.
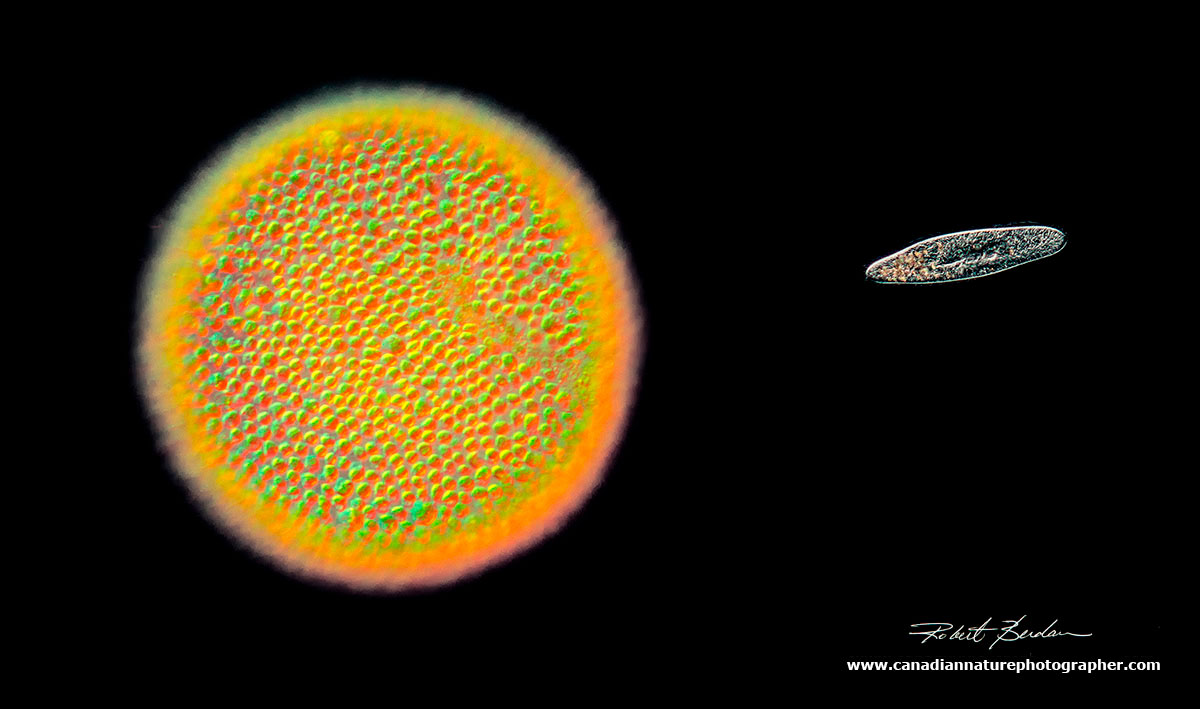
Using DIC and darkfield microscopy, The Volvox colony resembles the sun while the paramecium resembles a spaceship (DM). 250X.
Rotifers
There are about 2000 species of rotifers, most live in freshwater. They range in size from 40-200 microns (micron = 1\1000 of a millimetre). Most are predatory or suspension feeders. Rotifers have two conspicuous features 1) specialized ciliated region on the anterior end called a corona. Usually they have two concentric rings of cilia that move quickly hence they were once called "wheel animalcules". The cilia are used for locomotion and to collect food. A few species lack this corona. 2) All rotifers possess a muscular pharynx, termed a mastax that includes a set of jaws called trophi. The trophi or jaws are easy to spot in many of my photographs below and they move in a chewing fashion.
A few species of rotifer (about 100) occur in saline waters and also some inhabit the film of water covering mosses, lichens and liverworts. They will occupy any container that holds water including rain gutters and bird baths. Most rotifers are solitary, but about 25 species form colonies. In some species there are no males. Most rotifers reproduce parthenogenically, in other words the females have eggs that form more females. Males do appear briefly in some species to fertilize eggs. The males are usually smaller and may look very different.
Rotifers posses a perivascular cavity or pseudocoleum. They have no respiratory or circulatory system. Some of their cells are syncitial i.e. multinucleate and all animals exhibit eutely - they have a constant number of cells (900-1000). Other structures that may be present are cirri, sensory antenna and palps. They possess a simple brain and may have eye spots which act as photoreceptors.
The majority of rotifers shown in my pictures below belong to the class of Bdelloids consisting of about 450 species world wide. Males are assumed not to exist in this group. Identification of Bdelloid species can be difficult as it requires examination of minute details at high magnification. Many rotifers also have the ability to form cysts which can withstand dessication, and extreme temperatures. They feed on bacteria, ciliates, vorticella and other small protozoa and some will also feed on other rotifers. They move by a combination of stretching and crawling like an inch worm and they can zoom about using their corona cilia like a propeller. They are fascinating to watch and can be found in all types of fresh water in concentrations up to 1000 animals per litre and some sewage ponds up to 12000\L.
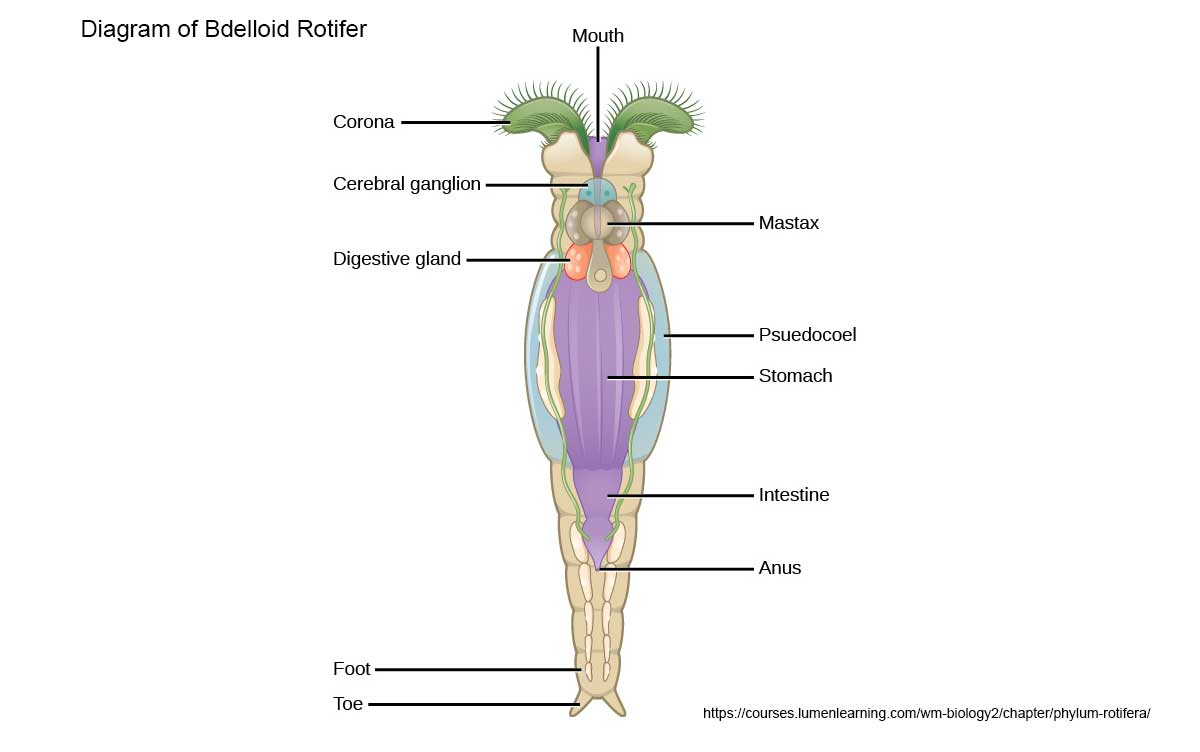
General diagram of a Bdelloid Rotifer - diagram source.

Pair of swimming Bdelloid rotifers with their coronas extended and their "wheels" of cilia used to propel them in the water. 200X DIC microscopy.

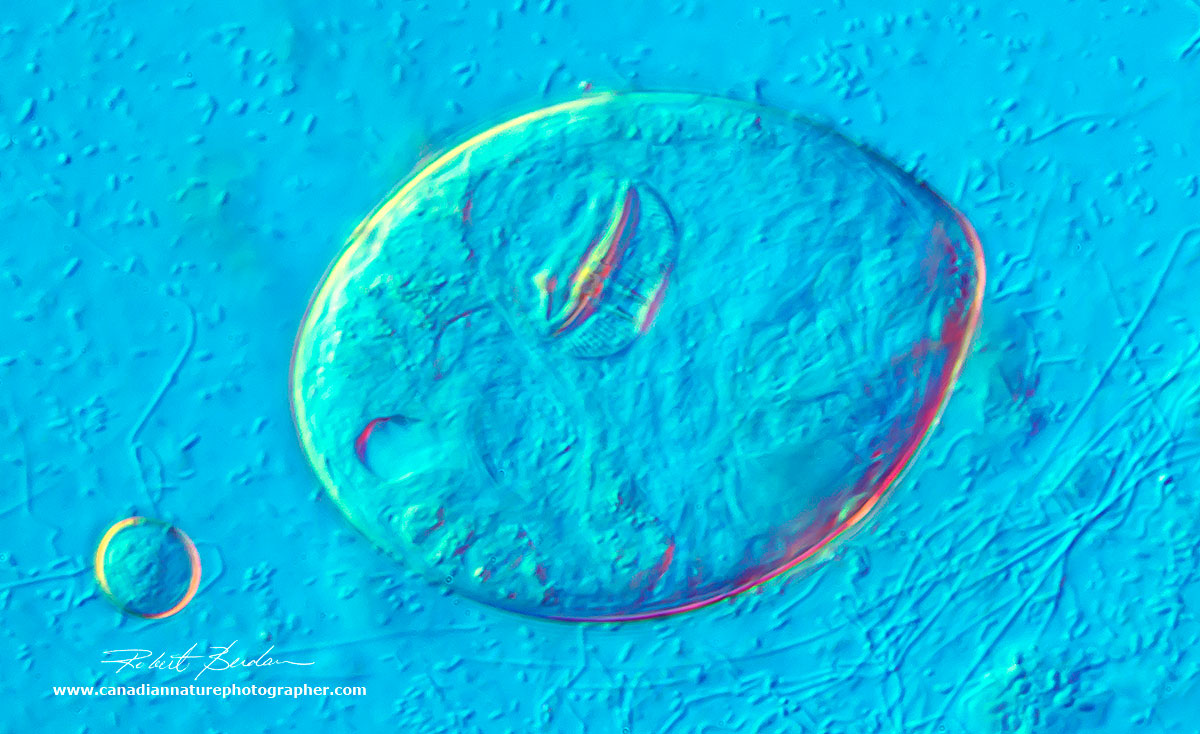
Single rotifer showing some of the internal organs, and jaws (trophi) near the center. 400X DIC microscopy.

A pack of Rotifers, like wolves head out in search of prey like the smaller ciliate on the right. 200X DIC.
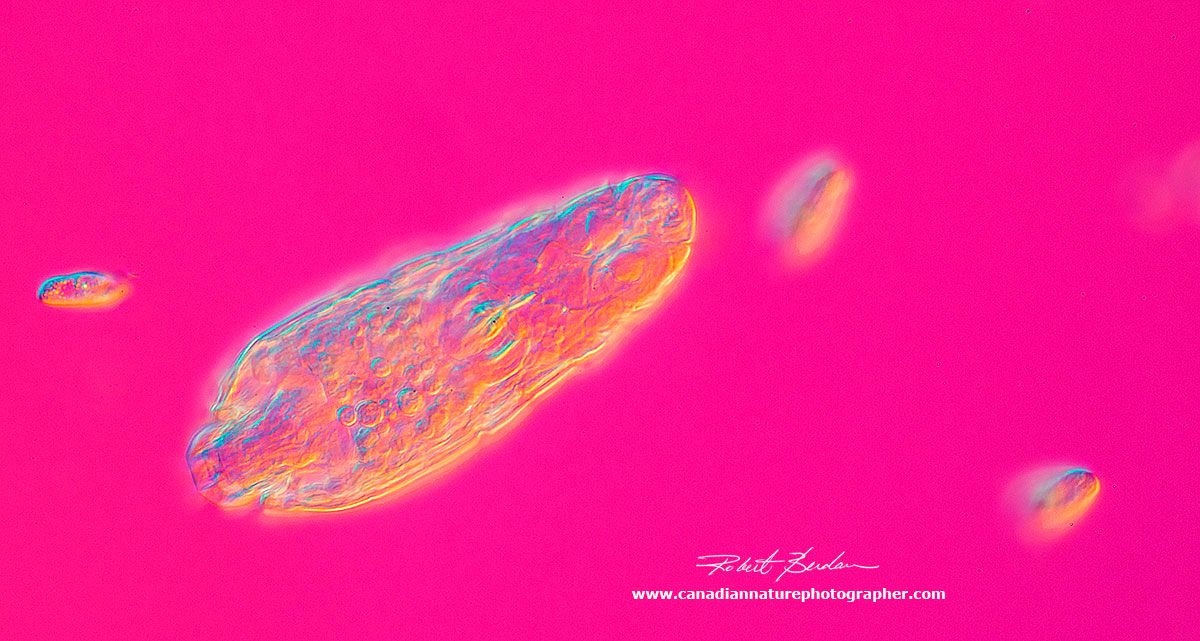
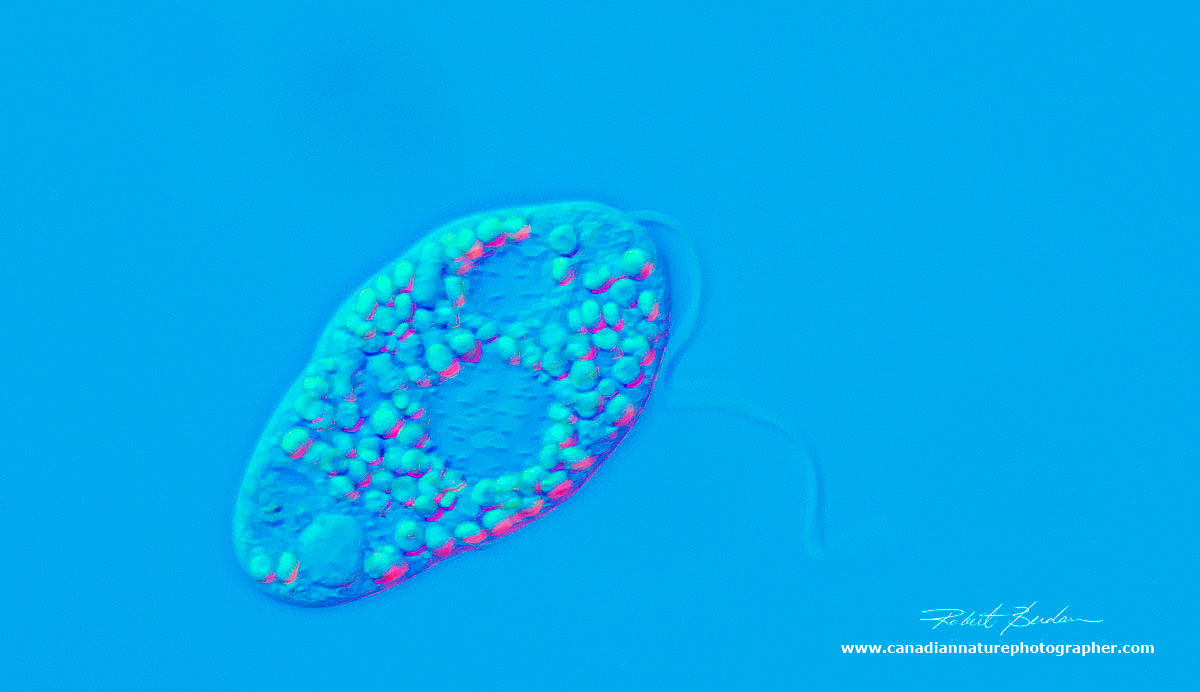
Single rotifer surrounded by 3 smaller euglena. The rotifer has contracted its body. DIC 200X.

Single rotifer stretched out, DIC microscopy 200X

Couple of rotifers, with clearly visible trophi - jaws. DIC microscopy 200X
Contracted Rotifer showing 2 pronged tail, note the Mastax inside.
Rotifer cyst DIC microscopy 400X. You can see part of the Mastax jaws inside.

Rotifer cyst (larger object) and on the right is a smaller Euglena 400X DIC microscopy

Extended Bdelloid rotifer showing dorsal antenna that sticks out of the top and can be retracted and it appears to be sensory in function, it may be a mechanoreceptor or chemical sensor. 200X DIC combined with Rheinberg lighting

Rotifer by Darkfield microscopy appears transparent, 200X

Scanning electron micro-graphs of Bdelloid rotifers, and their jaws by By Diego Fontaneto - Who Needs Sex (or Males) Anyway? Gross, L. PLoS Biology Vol. 5, No. 4, e99 doi:10.1371/journal.pbio.0050099, Learn more about Rotifers at Wikipedia.
Photomicrography Techniques I used
1. Focus stacking - the process involves taking several photos by varying the focus in about 1-2 micron increments. The animal most be holding still for this to work. I took between 2-10 photos and then stacked them in Photoshop or Helicon focus as I describe in an earlier article.
2. To slow animals down I let the water under the coverslip evaporate and or use a paper towel on the edge of the coverslip to draw water out thus pinning some of the larger organisms down so they can't move and so I can perform focus stacking. Most protozoa and rotifers move very quickly only amoeba seem to move more slowly. Other methods to slow the organisms down include adding methyl cellulose to the water, bubbling carbon dioxide through the water (use a straw and just blow for a few minutes) or adding a small concentration of alcohol.
3. Phase contrast, DIC, Darkfield, Rheinberg lighting, Polarizing microscopy are all methods used to view protozoa and rotifers and their intracellular organelles. In bright field microscopy most of these organisms appear transparent. You can stain the animals, but this often kills them and may obscure their internal components.
4. I edit, optimize my images and clean the backgrounds using Adobe Photoshop. Many of the slides contain debris, dirt, air bubbles that I remove digitally, but I do not alter the biological integrity of the animals.
5. Cameras used were the Nikon D500 and D800 attached to a Zeiss Axioscope Microscope with 10, 20, 40 and 63X Plan Achromat Infinity objectives. Camera settings included ISO 200-400, using live view and Digicam software (free to download) to save images and movies onto my Laptop.
6. Microscope camera adaptors are available starting at $20 to about $100 from AMScope. Other companies offer adaptors, but usually charge more. The biggest challenge is to elliminate vibration from the camera shutter, I use LView and\or mirror lockup. Some photographers use Afocal method with their cameras to reduce vibration.
7. An eyepiece reticule and micrometre slide with a small scale can be used measure an organisms size, and then determine the exact magnification. In this article I only provide approximate magnifications - view this PDF or watch some YouTube videos on how to measure objects using a microscope if interested.
8. You don't need an expensive microscope to view or photograph Pond life. A decent used microscope can be purchased for about $100 to $300 . Phase contrast will cost a few hundred dollars more. Darkfield, Rheinberg lighting can be added for a few dollars or the filters can be homemade. Unfortunately there are no cheap Interference microscopes. Microscope objectives also come in a variety of qualities from lowest to highest: Achromat, Plan Achromats, Semi-Apochromats and Apochromats which are the highest quality and also the most expensive. To learn more about microscope objectives search the Net or start here and try this link.
All of the animals shown in this article can be collected in local ponds, streams, lakes etc. If you are willing to break through the ice on a frozen pond they can be found in winter though they are not as numerous. You will need a microscope capable of 100-400X magnification to see them. You may be able to use a microscope from school or you can buy a used one for about $100-$300 by searching Kijjii, Ebay and government surplus. If purchasing a microscope I recommend you seek out someone that is experienced and can guide you in your purchase.
Video showing Protozoa and Rotifers
Watch Protozoa and Rotifers on Youtube (same video) - https://youtu.be/PtIJzX96lzs - includes sound
Note there is a significant loss of sharpness on the Youtube Video due to compression they perform.
 Zeiss Axioscope, Nikon D800 and laptop computer which was used to take pictures for this article.
Zeiss Axioscope, Nikon D800 and laptop computer which was used to take pictures for this article.
References:
Paramecium (1985) ed H. D. Gortz Springer Verlag, NY
The Biology of Stentor (1961) Vance Tartar, Pergamon Press, NY
Freshwater Invertebrates - Ecology and General Biology (2010) eds J.H. Thorp and D.C.Rogers, Academic Press,NY.
Freshwater Algae of North America (2015) J.D. Wehr et. al. eds , Academic Press. NY, 2nd edition.
Euglenoid Flagellates (1967) Gordon F. Leedale, Prentice Hall, NY
Plankton Rotifers Biology and Taxonomy (1974) A. Ruttner-Kolisko, Stuttgart, Germany
A. Örstan and M. Plewka (2017) An Introduction to Bdelloid Rotifers. Research Gate and available at www.quett.org
D. Fontaneto and WH. De Smet (2015) Chapter 4 Rotifera - research gate download PDF - comprehensive review
Microscopy from the very beginning - free book produced by Zeiss PDF
The Path of Photomicrography towards artistic recognition - download Essay as PDF
Websites:
Zeiss online microscopy
Boreal Science\Ward Science - source of the pond organisms
Quality Microscopes in Calgary - Zeiss dealer sells stereomicroscopes but may also have some light microscopes
Macrophotography.net - Forum on macro and photomicrography where you can ask for advice and learn.
UK Microscopy - great resource for anyone interested in microscopy & photomicrography
Rheinberg Filters and simulate DIC using Oblique lighting - this article has numerous useful references

My Zeiss Axioscope microscope with DIC optics, and Nikon D800 camera - Geek's paradise.
Note to teachers you have permission to use my pictures for teaching, I appreciate credit and\or link back to my web site if possible. For commercial use of my photos please contact me. The diagrams are freely available from Wikipedia or other web sites indicated for non-commercial purposes though you may be required to provide proper attribution to the author or source - please contact them directly if you would like to use the diagrams.
I provide training for anyone interested on how to use a microscope and\or how to take photomicrographs in my Calgary studio - if interested please contact me.
Authors Biography & Contact Information

Robert Berdan is a professional nature photographer living in Calgary, AB specializing in nature, wildlife and science photography. Robert offers photo guiding and private instruction in all aspects of nature photography and Adobe Photoshop training. Robert is also an adjunct assistant professor at the University of Calgary in the Department of Cell Biology and a member of the Univ. of Calgary Microscopy Interest Group.
Email at: rberdan@scienceandart.org
Web site: www.canadiannaturephotographer.com
Phone: MST 9am -7 pm (403) 247-2457.
Previous Articles by Robert Berdan on Microscopy
Home Microscopy Laboratory for Photomicrography
The Art & Science of Photomicrography with Polarized Light
Microscopy Life in Ponds and Rainwater - Pond Scum I
Photomicrographs of Diatoms from 1877 by John T. Redmayne
Focus Stacking comparing Photoshoop, Helicon Focus, Zerene
Photographing Microscope Plant and Animal Life - Pond Scum II
Scanning Electron Microscopy - Photography
Rheinberg Filters for Photomicrography
Photographing Through a Microscope - Photomicrography - Inner Space
Using a Flatbed and Slide Scanner as a low Power Microscope
Taking Pictures with a Microscope reveals Invisible Worlds
Click on the buttons below and share this site with your friends