
Photographing Water-fleas - Daphnia
by Dr. Robert Berdan
July 31, 2018 (updated April 25, 2019)

Water flea Ceriodaphnia frontal view by DIC (Differential Interference Contrast) microscopy. One micron = 1\1000 of a millimetre.
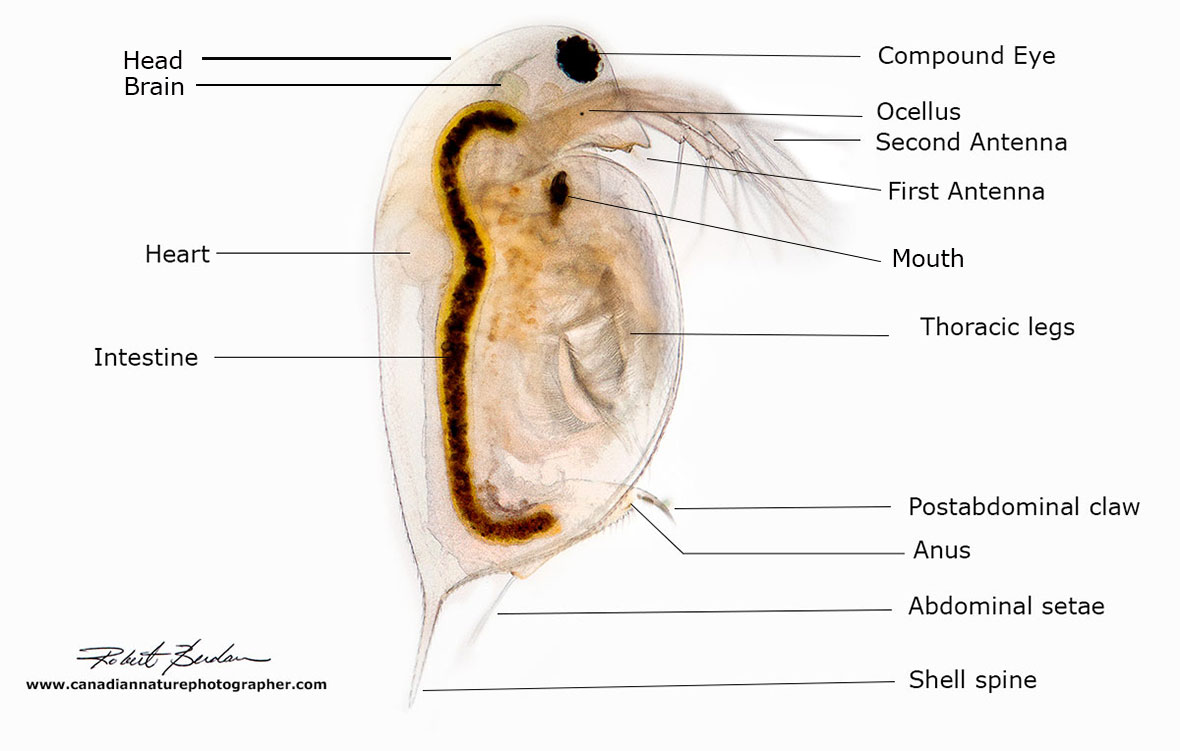
Photo-diagram of Daphnia also called a Water-flea viewed from the side 100X brightfield microscopy. They range in size from about 0.5 mm up to 6 mm (500-6000 microns).
Introduction
The English name for Daphnia, water-flea, originates from the jumping-like behaviour they exhibit while swimming. This is the result of the beating of the large secondary antenna which are used to swim. The rapid downbeat produces a quick upward movement while their high density causes them to sink (Ebert 2005). In many species the carapace is translucent making them excellent subjects for microscopic study. One can see the heart pumping (200 beats\minute at 20°C) and it changes with temperature. They have an open circulatory system and use hemoglobin as a pigment to hold oxygen. They are prone to alcohol intoxication and can be used to study the effects of the depressant on the nervous system and heart rate. One can also perform experiments with caffeine, nicotine or adrenaline and watch the effects on heart rate. I remember studying them in a first year University Biology lab to measure the effects of temperature on heart rate (see Khan and Kahn - PDF). They are also being used to study climate change on how increasing temperature affects their populations and size.

Side view of Daphnia Darkfield microscopy
Male Daphnia are smaller then females, but males have larger antennules and their first leg is armed with a hook used in clasping. Both males and females have a cuticular carapace composed of chitin a polysaccharide. They have an open circulatory system and use hemoglobin to carry oxygen. They produce more hemoglobin in anoxic environments and can appear red in colour.
Female Daphnia produce eggs primarily by parthenogenesis (without fertilization) during the most of the season. At the end of the season they can produce resting eggs encapsulated in a protective saddle-like structure called an ephippium which is strongly melanized and can contain 1 or 2 eggs. The ephippium is cast off on the next molt (see photo below in the section about eggs). These resting eggs are produced sexually and can survive winter, dessication and Ultraviolet radiation. Females are hatched from resting eggs which in turn produce more parthenogenetic eggs.
Daphnia are often the dominant zooplankton and serve as primary food source for other animals. They provide food for hydra, the phantom midge larvae Chaoborus, beetles like water boatman Notonecta, and a variety of small fish. Daphnia feed primarily on algae, bacteria and other small organisms. They can live up to 13-14 months in oligotrophic (low algal production) lakes, though typically they live for 5-6 months. Eggs hatch inside the female brood pouch where they remain for about 3 days before being released. The young undergo 4-6 instars (phase between two periods of molting) before they can reproduce. To learn more about Daphnia see some of the references at the end of this article and some of the Keys to identify them.

Frontal view of Ceriodaphnia - focus stack, Darkfield microscopy
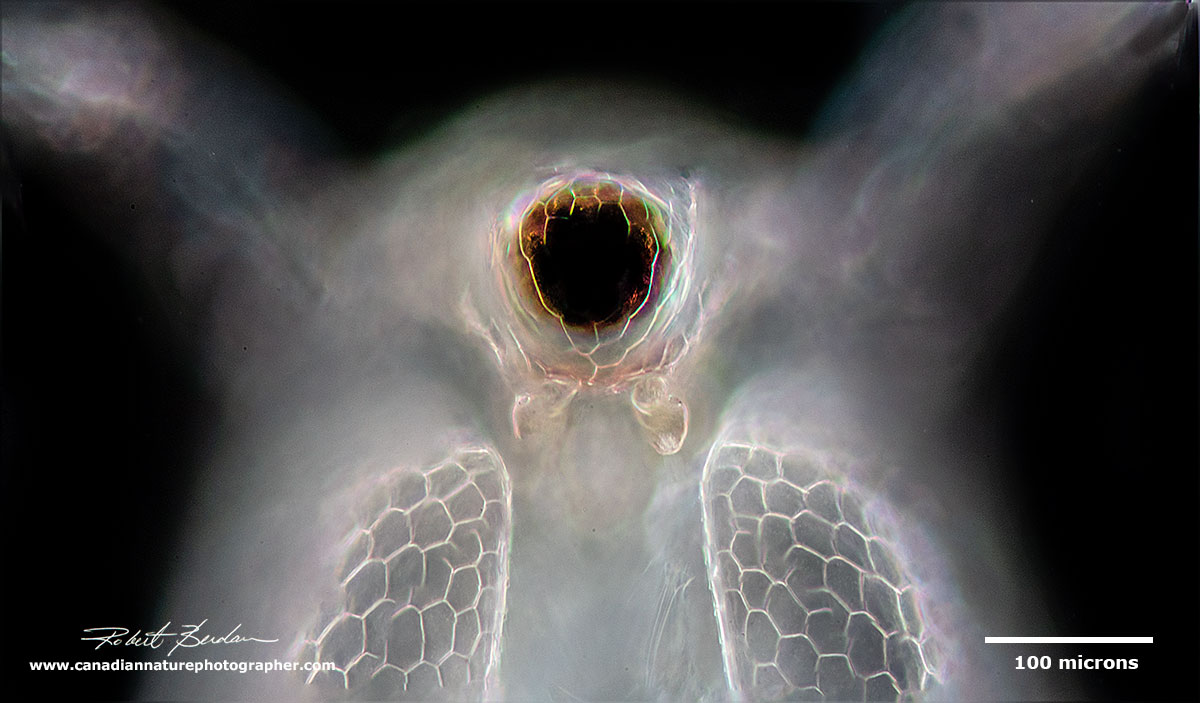
Frontal view showing the large compound eye and the pair of first antenna under the eye Ceriodaphnia
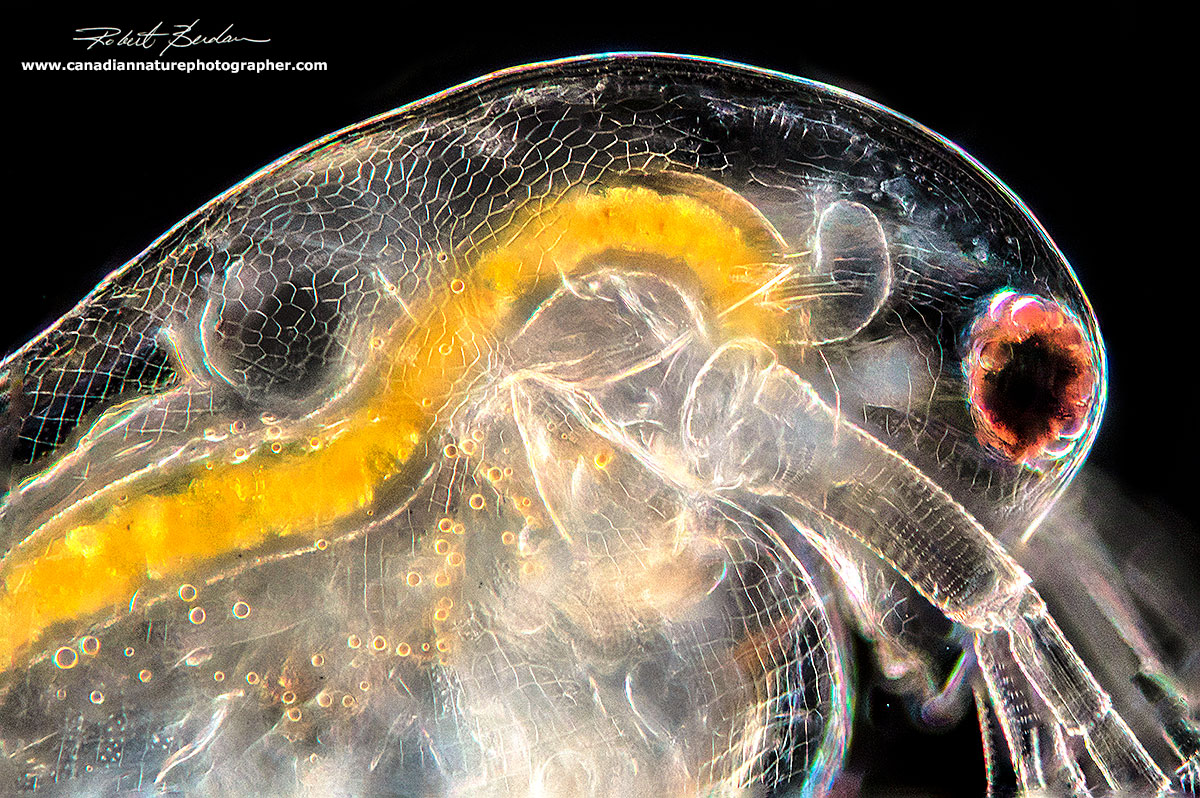
Darkfield microscopy showing Daphnia carapace texture and the translucence lets you see details of the organs. The heart is conspicuous and beats about 180-200 times a minute at 20°C.

Daphnia - side of the head showing the first antenna - it looks like several cigarettes. The large large compound eye is attached by muscle below it.
Eggs

Three Simocepahlus eggs are visible in this Darkfield microscopy image.
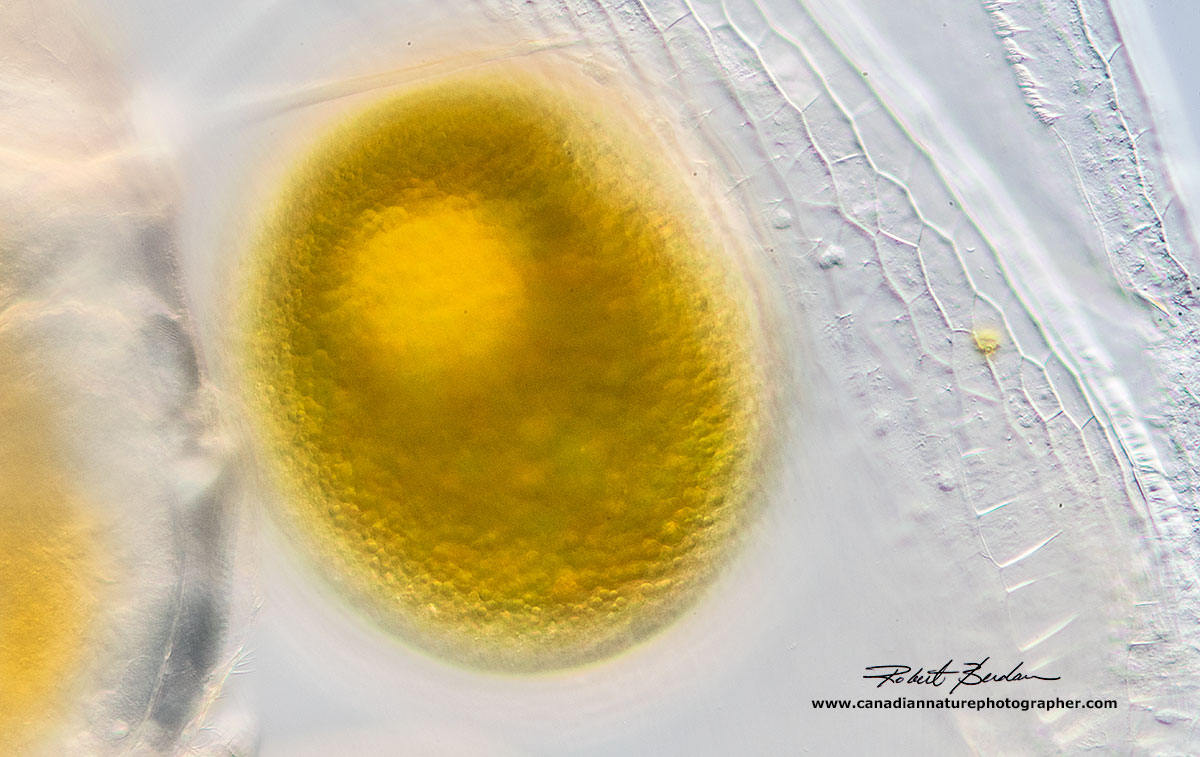
Single egg inside the Simochephalus carapace note the texture of the carapace walls
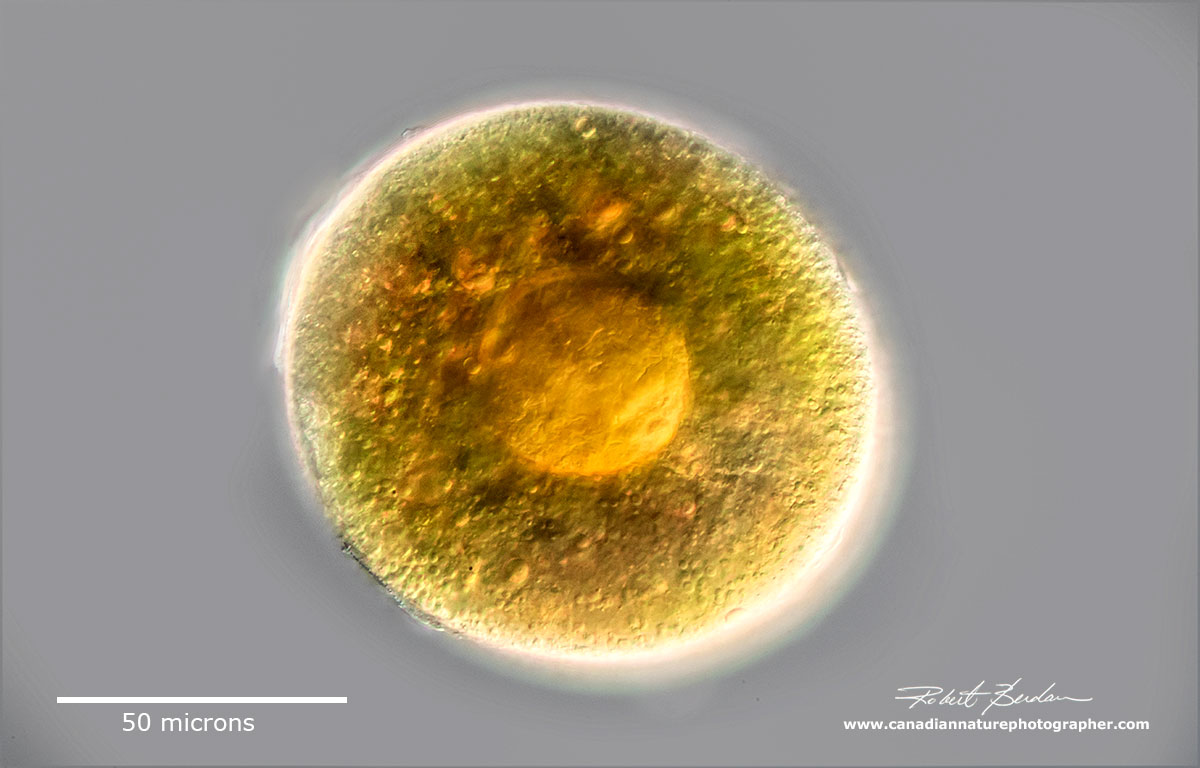
Single Daphnia egg extruded from the carapace

Ephippium with 2 resting eggs. The eggs are covered with a heavily melanized case to protect the eggs from UV and harsh weather conditions.
Parasites
The most common parasite I found growing on Daphnia was a peritrich ciliate Vorticella sp. There could be one to hundreds of Vorticella attached to the carapace. I also see them attached on other arthropods like ostracods and rotifers and even on dead carapaces after molting. See Ebert (2005) for more information about Daphnia parasites.

Frontal view of Simocephalis covered with Vorticella - DIC microscopy.

Darkfield microscopy showing some of the attached Vorticella on Simocephalus.

Simocephalus sp with Vorticella sp growing on its carapace.

Vorticella on the head of a Simocephalus stained with Lugol's iodine which turns them a red colour. Darkfield microscopy.

Vorticella sp growing on Simocephalus carapace DIC microscopy.

Side view of Simocephalus viewed with a polarizing microscope - only the heart and muscles attached to the 2nd antenna are strongly birefringent (glow in polarized light).

Polarizing microscopy - at higher magnification the muscles attached to the 2nd antenna are coloured - i.e. birefringent under polarized light.

Front view of Ceriodaphnia by Bright field microscopy - focus stack.

Side view of Simocephalus by bright field microscopy showing their translucent carapace.

Side view of Simocephalus by Darkfield microscopy
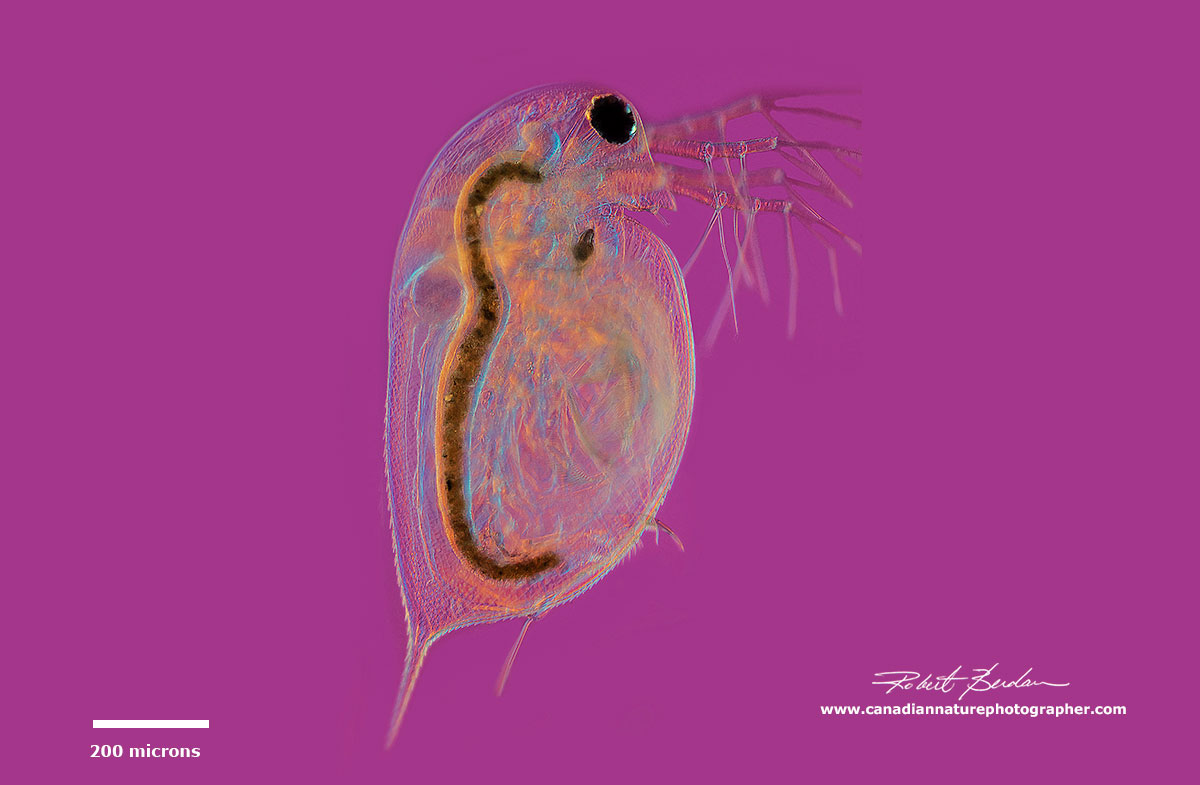
Side view of Daphnia sp by DIC microscopy
Taxonomy of Daphnia
Kingdom: Animalia
Phylum: Arthropoda
Class: Crustacea - body composed of segments e.g. crabs, lobsters, crayfish, shrimp etc
Order: Cladocera - small crustaceans called water fleas, with up to 10 pairs of appendages
Family: Daphniidae - water fleas
Genus: Daphnia - there are over 200 species of Daphnia, Daphnia pulex is the most common species. Species identification often requires close inspection of the postabdominal claw. Edmondson (1959 see references below) provides a key to common species.
Summary
Daphnia are common in most bodies of freshwater and provide an important food source for fish and other invertebrates. The are used in research to study climate change and as food for those that maintain aquaria. Their relatively large size and translucent carapace makes them ideal for studies using a microscope and for science projects.
Photomicrography
All photographs are of live Daphnia (except Lugol's iodine stained specimen) and were collected from fresh water - Frank Lake south of Calgary. For details on how I took these pictures please see my article - Tips on How to Take Better Pictures with a Microscope - Photomicrography. In brief, I used a Nikon D500 and Nikon D800 cameras, Zeiss Axioscope with DIC, Bright field, and Darkfield lighting and controlled my cameras with free software Digicam control. I held the Daphnia for photography by compression under the coverslip. Because Daphnia are relatively large I often used low magnification objectives 2.5, 5 and 10X in order to capture the entire animal. Focus stacking was often required in order to increase the depth of field in the photographs. See my article on focus stacking if you are interested in how I do this. RB
References
(2018) Daphnia - Wikipedia
(2016) Tiny Water Flea Provides Big Insight on Effects of Climate Change on Freshwater Organisms. Univ. Guelph web site at higher temp. the animals get smaller.
(2016) E. A. Angell How biotic interactions between Daphnia magna and Vorticella alter the ability of Daphnia magna to respond to abiotic changes. Undergraduate Honors Theses. Univ. Colorado - Download PDF
(2014) Epibionts and parasites on crustaceans... Oceanologia 56: 629-638. Download PDF
(2010) C.E. Cáceres and D. C. Rogers. Class Brachiopoda Chapt. 28 in Thorp and Covich's Freshwater Invertebrates 4th ed. pp 687-708. Academic Press, NY.
(2005) Ebert D. Ecology, Epidemiology, and Evolution of Parasitism in Daphnia. Bethesda, MD. Chapter 2 Introduction to Daphnia Biology - Download Chapter PDF or Download Book- PDF
(2004) Zooplankton - Guide to the Taxonomy Laurentian University - Taxonomy guide PDF
(2001) Daphnia Collecting Techniques and Discoveries Part I-4. H. Webb - www.microscopy-uk.org
(1959) W.T. Edmondson (ed) Fresh Water Biology 2nd edition, Cladocera by J.L. Brooks chapter 27. John Wiley. NY pg 587-656 - read online version free.
Investigating factors affecting the heart rate of Daphnia
The Amazing Daphnia water-flea - used as fish food, grow your own Daphnia
Daphnia culture and food can be purchased at Carolina.com - watch YouTube on heart rate
Daphnia bioassays for salt - Cornell University - projects for High School
Note to teachers and students you have permission to use my pictures for teaching or school projects. I appreciate credit and\or link back to my web site if possible. For any other use of my photos please contact me.
Acknowledgement: Two of the species Ceriodaphnia and Simocephalus were identified for me on macrophotography.net by Christian.
Authors Biography & Contact Information

Robert Berdan is a professional nature photographer living in Calgary, AB specializing in nature, wildlife and science photography. Robert retired from Cell\Neurobiology research to take up photography full time years ago. Robert offers photo guiding and private instruction in all aspects of nature photography and Adobe Photoshop training - including photomicrography, macrophotography.
Related Microscopy Articles by Robert Berdan on this web site
1. Photographing Gastrotrichs
2. Photographing Rotifers
3. Photographing Ciliates
4. Photographing Stentors - A Large Unicellular Protozoan (ciliate) living in Freshwater
5. How to Collect and Photograph Water Bears (Tardigrades).
6. Tips on How to take Better pictures with a Microscope
7. Microscopic Pond Organisms from Silver Springs Calgary
8. Microscopic Life in Ponds and Rainwater - Pond Scum I
9. Photographing Microscopic Plant and Animal Life - Pond Scum II
10. Photomicrography and Video of Protozoa, Volvox and Rotifers
11. Home Microscopy Laboratory for Photomicrography
12. The Art & Science of Photomicrography with Polarized Light
13. Photographing Through a Microscope Photomicrography - Inner Space
14. Focus Stacking comparing Photoshop, Helicon Focus and Zerene
15. Rheinberg Filters for Photomicrography
16. Scanning Electron Microscopy - Photography
17. Photomicrographs of Diatoms from 1877 by John T. Redmayne
Email at: rberdan@scienceandart.org
Web site: www.canadiannaturephotographer.com
Phone: MST 9am -7 pm (403) 247-2457.
Click on the buttons below and share this site with your friends